
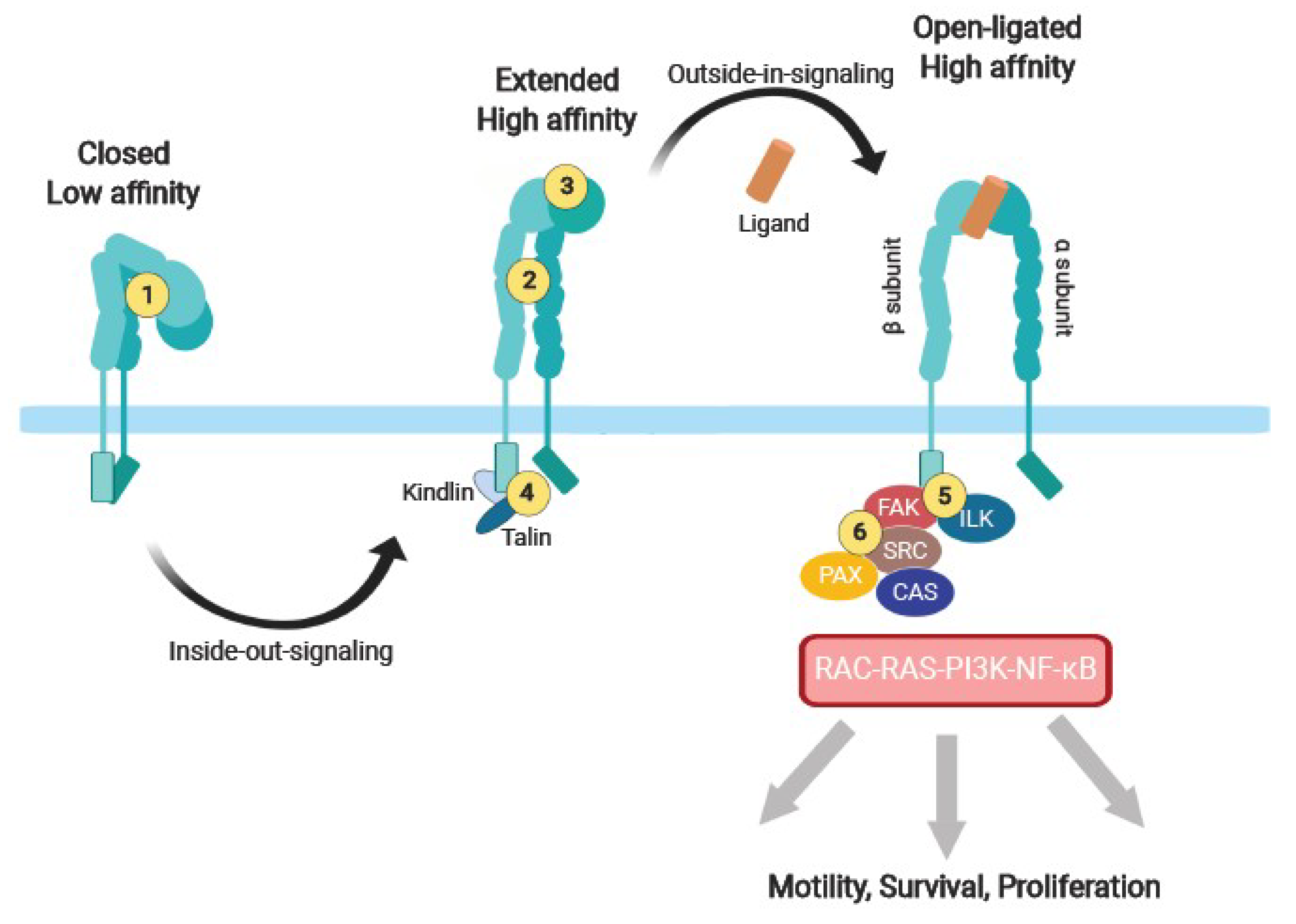
Activated Tie2 in these complexes stimulates promigratory and proliferative downstream pathways associated with FAK, Rac1, and ERK1/2 (MAPK) ( 10, 17). Interactions between α 5β 1 integrin and Tie2 ectodomains sensitize the receptor to activation by Ang1 and are enhanced in the presence of fibronectin (FN1) ( 10, 16, 17). However, understanding of the mechanism by which Ang2 switches between agonistic and antagonistic activities remains incomplete.īoth Tie receptors form complexes with the endothelial integrins α 5β 1 and α vβ 3 on the surface of cells. Specifically, the activation of junctional Tie2 by both Ang1 and Ang2 is enhanced when the receptor is associated with the Tie1 coreceptor and cleavage of Tie1 during inflammation is suspected to regulate the agonistic and antagonistic activities of Ang2 ( 16).
In some conditions, Ang2 may also function as a weak agonist of Tie2. In many disease states, the concentrations of the 2 angiopoietins are heavily skewed in favor of Ang2, which contributes to the progression of several vascular diseases through pathological increases in vessel permeability and instability ( 13– 15). Ang1 inhibition leads to reduced perivascular coverage and either vessel regression or angiogenesis in the absence or presence of other growth factors, respectively ( 11, 12). In contrast, angiopoietin 2 (Ang2) is essential for angiogenesis where it is rapidly released from Weibel-Palade bodies stored within ECs and destabilizes the vasculature by antagonizing the activities of Ang1. Alternatively, stimulation of Tie2 at the EC–extracellular matrix (EC-ECM) interface induces proliferation by ERK1/2 and migratory responses via Dok2 activities ( 8, 10). In quiescent vessels, paracrine release of angiopoietin 1 (Ang1) from perivascular cells stimulates Tie2 clusters across endothelial cell (EC) junctions, leading to the activation of the antiapoptotic Akt and reduced permeability through actin rearrangement and dephosphorylation of MLC2 by the Rap1 pathway ( 7– 9). 5, 6) has emerged as a major regulator of vascular homeostasis and a promising therapeutic target. The angiopoietin (Ang)/Tie system (reviewed in refs. IntroductionĮxcessive vascular growth, instability, and leakage are critical pathophysiological events in the progression of several diseases, including neovascular age-related macular degeneration (NVAMD), diabetic macular edema, cancer growth and metastasis, and uveitis and other inflammatory diseases ( 1– 4). Because Ang2 levels are very high in ischemic diseases, such as diabetic macular edema, neovascular age-related macular degeneration, uveitis, and cancer, targeting α 5β 1 with AXT107 provides a potentially more effective approach to treat these diseases. The potentiation of Tie2 activation by Ang2 even extended to mouse models in which AXT107 induced Tie2 phosphorylation in a model of hypoxia and inhibited vascular leakage in an Ang2-overexpression transgenic model and an LPS-induced inflammation model. These data suggest that α 5β 1 sequesters Tie2 in nonjunctional locations in endothelial cell membranes and that AXT107-induced disruption of α 5β 1 promotes clustering of Tie2 at junctions and converts Ang2 into a strong agonist, similar to responses observed when Ang1 levels greatly exceed those of Ang2. In the presence of Ang2 and AXT107, junctional Tie2 is activated, downstream survival signals are upregulated, F-actin is rearranged to strengthen junctions, and, as a result, endothelial junctional permeability is reduced. AXT107 disrupts α 5β 1 and stimulates the relocation of Tie2 and α 5 to cell junctions. Previously, AXT107 was shown to inhibit VEGFR2 and other growth factor signaling via receptor tyrosine kinase association with specific integrins. AXT107, a collagen IV–derived peptide, has strong antipermeability activity and has enabled the elucidation of this previously undetermined mechanism.
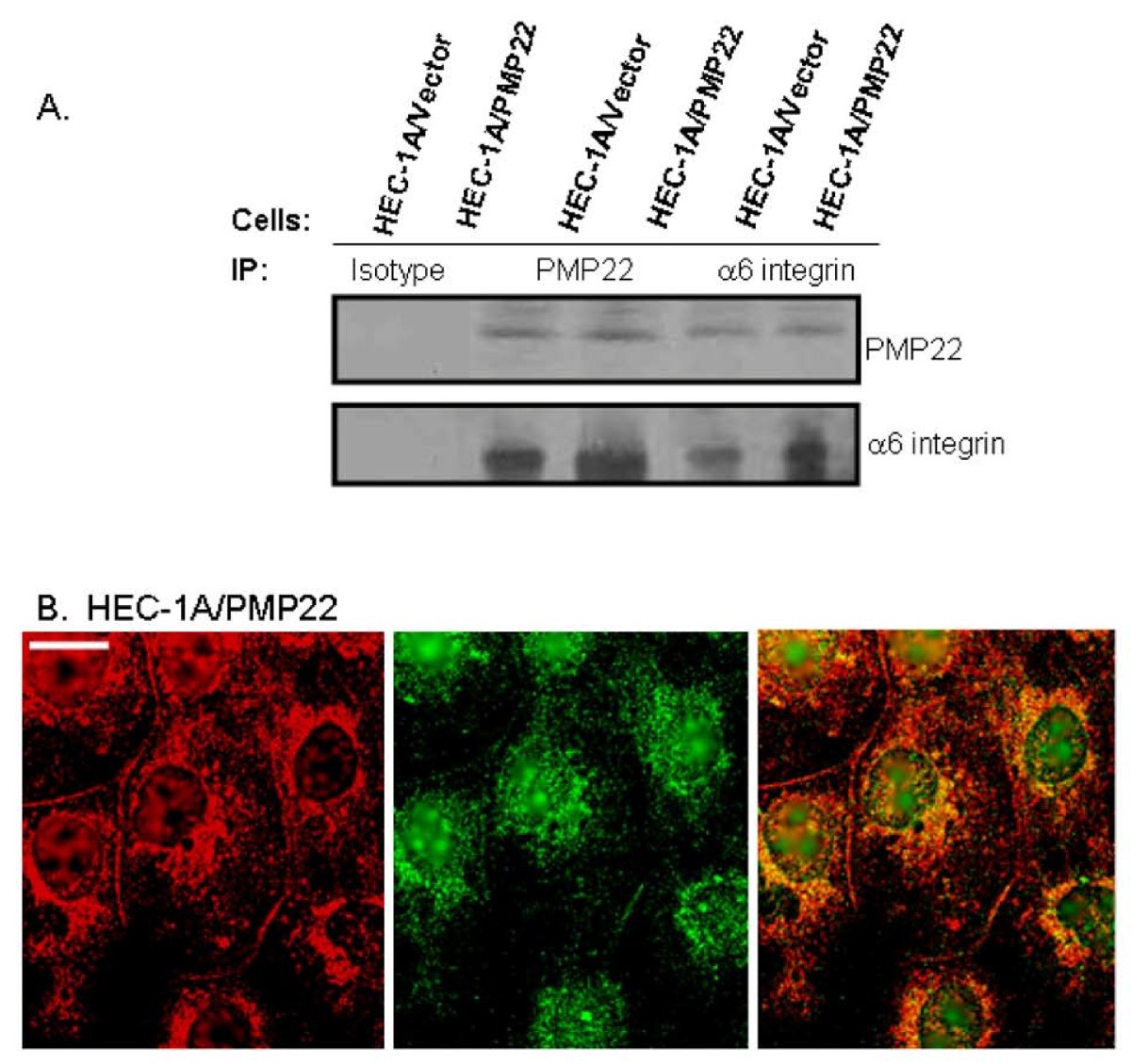
Interactions between Tie2 and α 5β 1 integrin have emerged as part of this control however, the mechanism is incompletely understood. The angiopoietin (Ang)/Tie2 signaling pathway is essential for maintaining vascular homeostasis, and its dysregulation is associated with several diseases.


 0 kommentar(er)
0 kommentar(er)
